RFP-VI: Peer Review
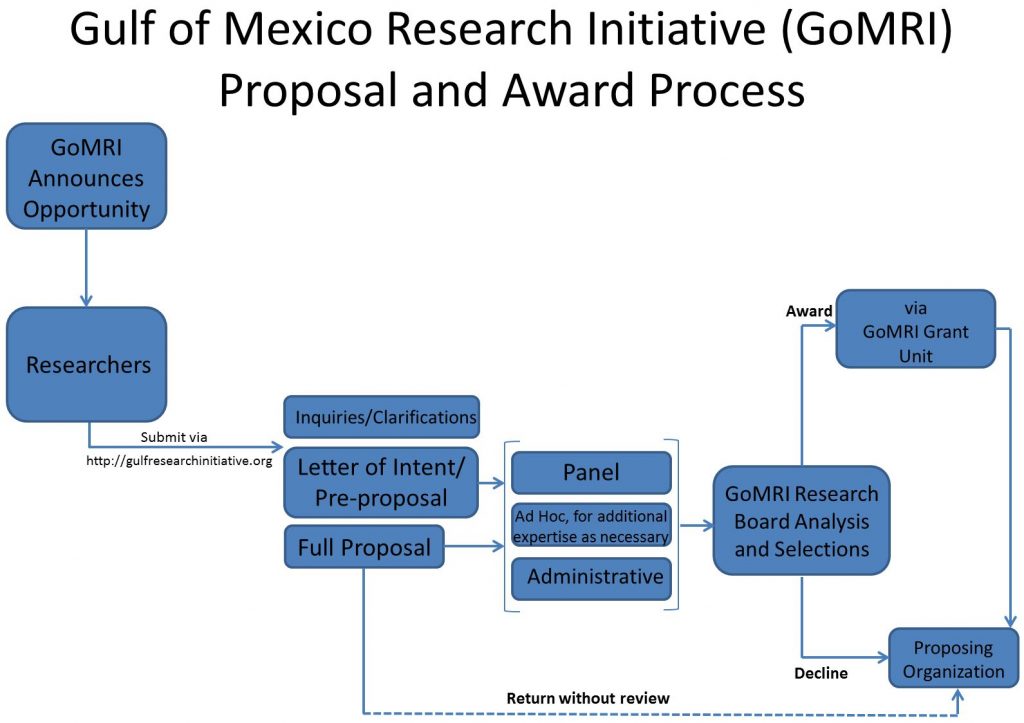
The Gulf of Mexico Research Initiative (GoMRI) will select awards for funding by merit review based on peer evaluation, modeled after the National Science Foundation’s Peer Evaluation Process. Letters of Intent will not be evaluated, but provide essential information for establishing peer review panels. An administrative review of the full proposal will take place to ensure that each proposal follows the proposal submission instructions, formatting guidelines, and includes all required sections. A panel of experts will evaluate the full proposals according to the review criteria listed in RFP-VI:
Scientific Value and Merit (80% for Individual Investigators; 70% for Consortia)
- Scope, quality, and potential for fundamentally significant results of the proposed research
- Project plan describing integration among any sub-projects
- Value of the anticipated research outcomes for contributing to the major research themes of the GoMRI
- Effective utilization or synthesis of research efforts completed or in progress under RFP-I through RFP-V
- A final research product that will be more than the sum of the results of the individual sub-projects
- Scope, quality, and potential of the proposed public education and outreach activities (Consortia only)
Qualifications (20% for Individual Investigators; 15% for Consortia)
- Expertise of PI / Consortium Director and co-PIs in the relevant research domains and in the delivery of focused research
- A demonstrated record of scientific achievement in the relevant science
- Realistic research capability, including ready availability of appropriate facilities, materials, equipment, and expertise for the duration of the project
- Knowledge of the Gulf of Mexico region
Management (15% for Consortia only)
- The feasibility to complete all research and analysis within the two years of the life of the award
- Governance and communication structure proposed to support effective exchange of ideas, data and results
- Data management policies, favorably including a strong record of previous submission of data to a public database (where applicable, of GoMRI data to the Gulf of Mexico Research Initiative Information & Data Cooperative, GRIIDC)
- Compliance with US research requirements (e.g., biosafety, animal research, ethics in science, conflicts of interest, etc.) and necessary safety training